- Biotic and abiotic stress,
- nanoparticles,
- silicon,
- performance,
- epidermis
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
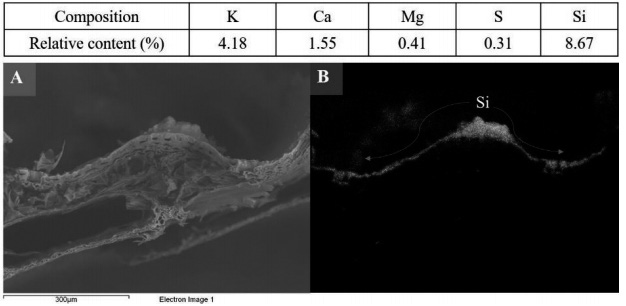
All technological innovation that influences research to achieve yields and counteract biotic and abiotic stress in crops should be a priority for governments and scientists around the world. Silicon nanoparticles (NpSi) in the production and protection of crops are used as a sustainable strategy. In addition to NpSi, other nanoparticles have been applicable in areas such as environmental remediation, medicine and smart sensors. There are plants that accumulate high concentrations of Si in their tissues, such as “horsetail” (Equisetum arvense). A recent analysis of the elemental composition of E. arvense in a cross section, epidermis, and total biomass indicated that the Si concentration was higher in comparison with macro and micronutrients. Elemental mapping showed that all polymerized silicon (SiO2 · nH2O) is available in the epidermis of Equisetum. Currently, our team is investigating the extraction, purification and quantification of SiNp. The lines of emerging research should be those related to the interaction of SiNp in the cell wall, concentration and intelligent application with aerial equipment in crops such as vegetables, cereals, and fruits.
References
- M. Luyckx, J. F. Hausman, S. Lutts, G. Guerriero, Front Plant Sci 8, 411, (2017).
- H. Etesami, B. R. Jeong, Ecotoxicol Environ Saf 147, 881-896, (2018).
- O. Markovich, E. Steiner, Š. Kouřil, P. Tarkowski, A. Aharoni, R. Elbaum, Plant Cell Environ 40, 1189-1196, (2017).
- R. Prasad, A. Bhattacharyya, Q. D. Nguyen, Front Microbiol 8, 1014, (2017).
- J. Montpetit, J. Vivancos, N. Mitani-Ueno, N. Yamaji, W. Rémus-Borel, F. Belzile, R. R. Bélanger, Plant Mol Biol 79, 35-46, (2012).
- J. Sangeetha, D. Thangadurai, R. Hospet, E. R. Harish, P. Purushotham, M. A. Mujeeb, S. B. Araneda, Nanotechnology. Springer, Singapore, (2017).
- E. Epstein, Proc Natl Acad Sci U S A 91, 11-17, (1994).
- D. Debona, F. A. Rodrigues, L. E. Datnoff, Annu Rev Phytopathol 55, 85-107, (2017).
- R. J. Haynes, Advances in Agronomy. Academic Press, (2017).
- J. F. Ma, N. Yamaji, Trends in plant science 11, 392-397, (2006).
- C. J. Prychid, P. J. Rudall, M. Gregory, Bot Rev 69, 377-440, (2003).
- N. Mitani, J. F. Ma, T. Iwashita, Plant Cell Physiol 46, 279-283, (2005).
- J. F. Ma, N. Yamaji, Trends in Plant Science 20, 435-442, (2015).
- M. J. Hodson, P. J. White, A. Mead, M. R. Broadley, Ann Bot 96, 1027- 1046, (2005).
- H. A. Currie, C. C. Perry, Annals of Botany 100, 1383-1389, (2007).
- A. Frew, L. A. Weston, O. L. Reynolds, G. M. Gurr, Ann Bot 121, 1265- 1273, (2018).
- Zaid, F. Gul, M. A. Ahanger, P. Ahmad, Metabolites and Regulation Under Environmental Stress, (2018).
- M. A. Limmer, J. Mann, D. C. Amaral, R. Vargas, A. L Seyfferth, Sci Total Environ 624, 1360-1368, (2018).
- M. Rizwan, S. Ali, M. Ibrahim, M. Farid, M. Adrees, S. A. Bharwana, F. Abbas, Environ Sci Pollut Res Int 22, 15416-15431, (2015).
- S. Muneer, Y. G. Park, A. Manivannan, P. Soundararajan, B. R. Jeong, Int J Mol Sci 15, 21803-21824, (2014).
- Yin, L., Wang, S., Li, J., Tanaka, K., Oka, M. Acta Physiol Plant 35, 3099- 3107, (2013).
- Y. H. Kim, A. L. Khan, M. Waqas, J. K. Shim, D. H. Kim, K. Y. Lee, I. J. Lee, J. Plant Growth Regul 33, 137-149, (2014).
- Meharg, A. A. Meharg, Environ Exp Bot 120, 8-17, (2015).
- Y. Liang, W. Sun, Y. G. Zhu, P. Christie, Environ Pollut 147, 422-428, (2007).
- W. Chen, X. Yao, K. Cai, J. Chen, Biol Trace Elem Res 142, 67-76, (2011).
- K. P. V. da Cunha, C. W. A. do Nascimento, Water Air Soil Pollut 197, (1-4), 323, (2009).
- M. R. Romero-Aranda, O. Jurado, J. Cuartero, J Plant Physiol 163, 847- 855, (2006).
- Y. Liang, J. Zhu, Z. Li, G. Chu, Y. Ding, J. Zhang, W. Sun, Environ Exp Bot 64, 286-294, (2008).
- H. Miao, X. G. Han, W. H. Zhang, Ann Bot 105, 967-973l, (2010).
- H. F. Bakhat, N. Bibi, Z. Zia, S. Abbas, H. M. Hammad, S. Fahad, S. Saeed, Crop Prot 104, 21-34, (2018).
- Abdel Latef, A. A., & Tran, L. S. P, Frontiers in plant science 7, 243, (2016).
- U. Sienkiewicz-Cholewa, J. Sumisławska, E. Sacała, M. Dziągwa-Becker, R. Kieloch, Acta Physiol Plant 40, 54, (2018).
- T. Abbas, A. Sattar, M. Ijaz, M. Aatif, S. Khalid, A. Sher, Hortic Environ Biotechnol 58, 342-349, (2017).
- R. Hajiboland, N. Moradtalab, Z. Eshaghi, J. Feizy, Newzeal J Crop Hort 46, 144-161, (2018).
- T. Rangwala, A. Bafna, N. Vyas, R. Gupta, Indian J Agr Sci 52, 9-15.
- K. Tripathi, S. Singh, V. P. Singh, S. M. Prasad, D. K. Chauhan, N. K. Dubey, Front Env Sci 4, 46, (2016).
- Epstein, Ann Appl Biol 155, 155-160, (2009).
- J. Pozo, M. Urrestarazu, I. Morales, J. Sánchez, M. Santos, F. Dianez, J. E. Álvaro, HortScience 50, 1447-1452, (2015).
- M. Wang, L. Gao, S. Dong, Y. Sun, Q. Shen, S. Guo, Front Plant Sci 8, 701, (2017).
- R. M. R. N. K. Ratnayake, M. Y. U. Ganehenege, H. M. Ariyarathne, W. A. M. Daundasekera, Ceylon J Sci 47, 49-55, (2018).
- R. Suriyaprabha, G. Karunakaran, R. Yuvakkumar, P. Prabu, V. Rajen¬dran, N. Kannan, J Nanopart Res 14, 1294, (2012).
- M. H. Siddiqui, M. H. Al-Whaibi, Saudi J Biol Sci 21, 13-17, (2014).
- M. H. Siddiqui, M. H. Al-Whaibi, M. Firoz, M. Y. Al-Khaishany, Spring¬er, Cham. pp. 19-35, (2015).
- M. Janmohammadi, T. Amanzadeh, N. Sabaghnia, V. Ion, Bot Lith 22, 53-64, (2016).
- Laane, H. M. Plants 7, (2018).
- N. Dasgupta, S. Ranjan, C. Ramalingam, Environ Chem Lett 15, 591-605, (2017).
- J. S. Duhan, R. Kumar, N. Kumar, P. Kaur, K. Nehra, S. Duhan, Biotech¬nol Rep 15, 11-23, (2017).
- Mastronardi, P. Tsae, X. Zhang, C. Monreal, M. C. DeRosa, Springer, Cham, (2015).
- S. M. Rodrigues, P. Demokritou, N. Dokoozlian, C. O. Hendren, B. Karn, M. S. Mauter, P. Welle, Environ Sci Nano 4, 767-781, (2017).
- T. A. Shalaby, Y. Bayoumi, N Abdalla, H. Taha, T. Alshaal, S. Shehata, H. El-Ramady, Springer, Cham. pp. 283-312, (2016).
- A. Ditta, M. Arshad, M. Ibrahim, Nanotechnology and plant sciences. Springer, Cham, (2015).
- R. Raliya, V. Saharan, C. Dimkpa, P. Biswas, J Agric Food Chem, (2017).
- Shukla, P. K., Misra, P., Kole, C. In Plant Nanotechnology Springer, Cham, (2016).
- P. Wang, E. Lombi, F. J. Zhao, P. M. Kopittke, Trends Plant Sci 21, 699- 712, (2016).


