KINETICS STUDIES ON THE TAUTOMERIC REACTION OF 4-AMINO-5-METHYL-2,4-DIHYDRO-3H-1,2,4- TRIAZOLE-3-THIONE IN THE GAS PHASE: DFT AND CBS-QB3 METHODS USING TRANSITION STATE THEORY
- Isomerization,
- Rate constant,
- Reaction mechanism,
- Chemical kinetics,
- DFT
- NBO ...More
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
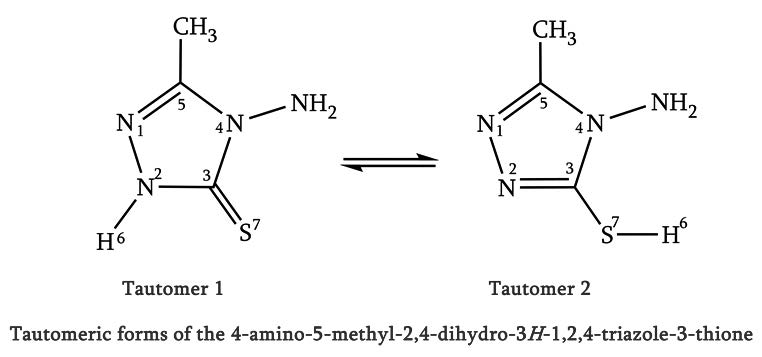
The isomerization reactions of the 4-amino-5-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione have been studied theoretically using density functional theory (DFT) along with various exchange-correlation functionals (B3LYP and M06-2x) as well as the benchmark CBS-QB3 quantum chemical approach. The calculated energy profile has been supplemented with calculations of kinetic rate constants by means of transition state theory (TST).
Based on the optimized isomers geometries using the CBS-QB3 method, a natural bond orbital analysis reveals that the electronic delocalization from non-bonding lone-pair orbitals [LP(e)S7] to the neighboring σ* N2-C3 antibonding orbital increase from isomer 1 to isomer 2. Also, the LP(e)S7→σ* N2-C3 delocalizations could fairly explain the increase of occupancies of LP(e) non-bonding orbitals in the ring of isomers 1 and 2 (2 > 1). The electronic delocalization from LP(e)S7 non-bonding to σ* N2-C3 antibonding orbitals increase the ground state structure stability, Therefore, the increase of LP(e)S7→σ*N2-C3 delocalizations could fairly explain the kinetics of the isomerization reactions 1 and 2 (k2 > k1). NBO results also suggest that the kinetics of these processes are controlled by LP→σ* resonance energies.
References
- B. Namratha, and S. L. Gaonkar, Int. J. Pharm. 6, 73 (2014).
- S. Nekkanti, R. Tokala, and N. Shankaraiah, Curr. Med. Chem. 24, 2887 (2017).
- J. K. Sahu, S. Ganguly, and A. Kaushik, Chin. J. Nat. Med. 11, 456 (2013).
- T. V. Ghochikyan, M. A. Samvelyan, A. S. Galstyan, and S. V. Grigoryan, Chem. Biol. 2, 8 (2016).
- G. A. Sandip, R. M. Suleman, and S. P. Vandana, Chem. Asian J. 6, 2696 (2011).
- P. Ratchanok, P. Veda, M. Prasit, C. Nantasenamat, S. Prachayasittikul, and S. Ruchirawat, Bioorg Med Chem. 23, 3472 (2015).
- R. Kaur, A. R. Dwivedi, B. Kumar, and V. Kumar, Anticancer. Agent. Med. Chem. 16, 465 (2016).
- A. N. Syed, M. Gurumurthy, J. C. Swarup, and P. Debashisha, Adv. Pharm. Res. 1, 26 (2010).
- J. Zhong, H. Aihong, L. Tao, H. Yan, L. Jianbing, and F. Jianxin, J. Organomet. Chem. 690, 1226 (2005).
- P. Elzbieta, and K. Iwona, II Farmaco 58, 423 (2003).
- S. Nadeem, and A. Waquar, Eur. J. Med. Chem. 45, 1536 (2010).
- J. Chen, X. Y. Sun, K. -Y. Chai, J. -S. Lee, M. S. Song, and Z. S. Quan, Bioorg. Med. Chem. 15, 6775 (2007).
- M. A. Ashraf, M. Hamdy, R. Abdel, S. A. Gamal-Eldien, and A. E. Mahamoud, Eur. J. Med. Chem. 44, 117 (2009).
- B. Tozkoparan, N. Gokhan, G. Aktay, E. Yesilada, and M. Ertan, Eur. J. Med. Chem. 35, 743 (2000).
- K. S. Bhat, B. Poojary, D. J. Prasad, P. Naik, and B. S. Holla, Eur. J. Med. Chem. 44, 5066 (2009).
- B. S. Holla, B. Veerendra, M. K. Shivananda, and B. Poojary, Eur. J. Med. Chem. 38, 759 (2003).
- C. Alkan, Y. Tek, and D. Kahraman, Turk. J. Chem. 35, 769 (2011).
- J. M. Vega-Pérez, I. Periñán, M. Argandoña, M. Vega-Holm, C. Palo- Nieto, E. Burgos- Morón, M. López-Lázaro, C. Vargas, J. J. Nieto, and F. Iglesias-Guerra, Eur. J. Med. Chem. 58, 591 (2012).
- J. Yao, J. Chen, Z. He, W. Sun, and W. Xu, Bioorg. Med. Chem. 20, 2923 (2012).
- C. S. Shantharam, V. D. M. Suyoga, R. Suhas, M. B. Sridhara, and G. D. Channe, Eur. J. Med. Chem. 60, 325 (2013).
- A. P. Keche, G. D. Hatnapure, R. H. Tale, A. H. Rodge, and V. M. Kamble, Bioorg. Med. Chem. Lett. 22, 6611 (2012).
- J. R. Burgeson, A. L. Moore, J. K. Boutilier, N. R. Cerruti, D. N. Gharaibeh, C. E. Lovejoy, S. M. Amberg, D. E. Hruby, S. R. Tyavanagimatt, R. D. Allen, and D. Daisar, Bioorg. Med. Chem. Lett. 22, 4263 (2012).
- B. Kocyigit-Kaymakcioglu, A. O. Celen, N. Tabanca, A. Ali, S.I. Khan, I. K. Khan, and D. E. Wedge, Molecules 18, 3562 (2013).
- S. Saha, D. Dhanasekaran, S. Chandraleka, N. Thajuddin, and A. Panneerselvam, Adv. Biol. Res. 4, 224 (2010).
- R. Casasnovas, J. Frau, J. Ortega-castro, A. Salva, J. Donoso, and F. Munoz, Int. J. Quantum. Chem. 110, 323 (2010).
- J. A. Montgomery, Jr., M. J. Frisch, J. W. Ochterski, and G. A. Petersson, J. Chem. Phys. 110, 2822 (1999).
- J. W. Ochterski, G. A. Petersson, and J. A. Montgomery, Jr., J. Chem. Phys. 104, 2598 (1996).
- N. R. Nyden, and G. A. Petersson, J. Chem. Phys. 75, 1843 (1981).
- G. A. Petersson, A. Bennett, T. G. Tensfeld, M. A. Al-Laham, W. Shirley, and J. Mantzaris, J. Chem. Phys. 89, 2193 (1988).
- G. A. Petersson, and M. A. Al-Laham, J. Chem. Phys. 94, 6081 (1991).
- G. A. Petersson, A. K. Yee, and A. Bennett, J. Chem. Phys. 83, 5105 (1983).
- J. A. Montgomery, Jr., J. W. Ochterski, and G. A. Petersson, J. Chem. Phys. 101, 5900 (1994).
- J. A. Montgomery, Jr., M. J. Frisch, J. W. Ochterski, and G. A. Petersson, J. Chem. Phys. 112, 6532 (2000).
- C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
- A. D. Becke, J. Chem. Phys. 98, 5648 (1993).
- Y. Zhao, and D. G. Truhlar, Acc. Chem. Res. 41, 157 (2008).
- T. H. Dunning, Jr., J. Chem. Phys. 90, 1007 (1989).
- H. Eyring, J. Chem. Phys. 3, 107 (1935).
- H. S. Johnston, Gas Phase Reaction Rate Theory, The Roland Press Co., New York, 1966.
- K. J. Laidler, Theories of Chemical Reaction Rates; McGraw-Hill: New York, 1969.
- R. E. Weston, and H. A. Schwartz, Chemical Kinetics, Prentice-Hall, New York, 1972.
- D. Rapp, Statistical Mechanics, Holt, Reinhard, and Winston, New York, 1972.
- E. E. Nikitin, Theory of Elementary Atomic and Molecular Processes in Gases, Claredon Press, Oxford, 1974.
- I. W. M. Smith, Kinetics and Dynamics of Elementary Gas Reactions, Butterworths, London, 1980.
- A. E. Reed, R. B. Weinstock, and F. Weinhold, J. Chem. Phys. 83, 735 (1985).
- J. K. Badenhoop, and F. Weinhold, Int. J. Quantum. Chem. 72, 269 (1999).
- Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT, 2009.
- I. I. R. Dennington, T. Keith, J. Millam, K. Eppinnett, W. L. Hovell, and R. Gilliland, GaussView, Version 3.09, Semichem, Inc.: Shawnee Mission, KS, 2003.
- Y. Zhao, and D. G. Truhlar, Theor. Chem. Acc. 120, 215 (2008).
- E. Zahedi, S. Shaabani, and A. Shiroudi, J. Phys. Chem. A 121, 8504 (2017).
- J. A. Sousa, P. P. Silva, A. E. H. Machado, M. H. M. Reis, L. L. Romanielo, and C. E. Hori, Braz. J. Chem. Eng. 30, 83 (2013).
- X. Li, and M. J. Frisch, J. Chem. Theory Comput. 2, 835 (2006).
- F. Fukui, J. Phys. Chem. 74, 4161 (1970).
- A. R. Oliaey, A. Shiroudi, E. Zahedi, and M. S. Deleuze, React. Kinet. Mech. Cat. 124, 27 (2018).
- S. Canneaux, F. Bohr, and E. Henon, J. Comput. Chem. 35, 82 (2013).
- M. D. Allendorf, T. M. Besmann, R. J. Kee, and M. T. Swihart, Chemical Vapor. Deposition: Precursors, Processes and Applications, 1st edn., The Royal Society of Chemistry, UK, 2009.
- K. A. Holbrook, M. J. Pilling, and S. H. Robertson, Unimolecular Reactions, 2nd edn., Wiley, Chichester, 1996.
- E. Wigner, J. Chem. Phys. 5, 720 (1937).
- E. P. Wigner, Z. Phys. Chem. B 19, 203 (1932).
- H. Eyring, S. H. Lin, and S. M. Lin, Basic Chemical Kinetics, Wiley, New York, 1980.
- A. Shiroudi, E. Zahedi, A. R. Oliaey, and M. S. Deleuze, Chem. Phys. 485–486, 140 (2017).
- H. Ostovari, E. Zahedi, I. Sarvi, and A. Shiroudi, Monatsh. Chem. 149, 1045 (2018).
- C. Eckart, Phys. Rev. 35, 1303 (1930).
- R. L. Brown, J. Res. Natl. Bur. Stand. 86, 357 (1981).
- A. Shiroudi, and M. S. Deleuze, Comput. Theor. Chem. 1074, 26 (2015).
- Computational chemistry comparison and benchmark database, pre-computed vibrational scaling factors. http://cccbdb.nist.gov/vibscalejust.asp
- J. Troe, J. Chem. Phys. 66, 4758 (1977).
- G. S. Hammond, J. Am. Chem. Soc. 77, 334 (1953).
- N. Agmon, and R. D. Levine, Chem. Phys. Lett. 52, 197 (1977).
- S. W. Benson, The Foundations of Chemical Kinetics, McGraw-Hill, New York, 1960.
- H. E. O’Neal, and S. W. Benson, S. J. Phys. Chem. 71, 2903 (1967).
- S. W. Benson, Thermochemical Kinetics, John Wiley & Sons, McGraw- Hill, New York, 1960.
- G. Lendvay, J. Phys. Chem. 93, 4422 (1989).
- A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev. 88, 899 (1988).
- K. B. Wiberg, Tetrahedron 24, 1083 (1968).
- A. E. Reed, J. E. Carpenter, and F. Weinhold, NBO version 3.1, 2003.
- A. Moyano, M. A. Periclas, and E. Valenti, J. Org. Chem. 54, 573 (1989).
- F. Rosas, R. M. Dominguez, M. Tosta, J. R. Mora, E. Marquez, T. Cordova, and G. Chuchani, J. Phys. Org. Chem. 23, 743 (2010).
- E. Marquez, J. R. Mora, T. Cordova, and G. Chuchani, J. Phys. Chem. A 113, 2600 (2009).
- J. E. Carpenter, and F. Weinhold, J. Mol. Struct. (THEOCHEM) 169, 41 (1988).


